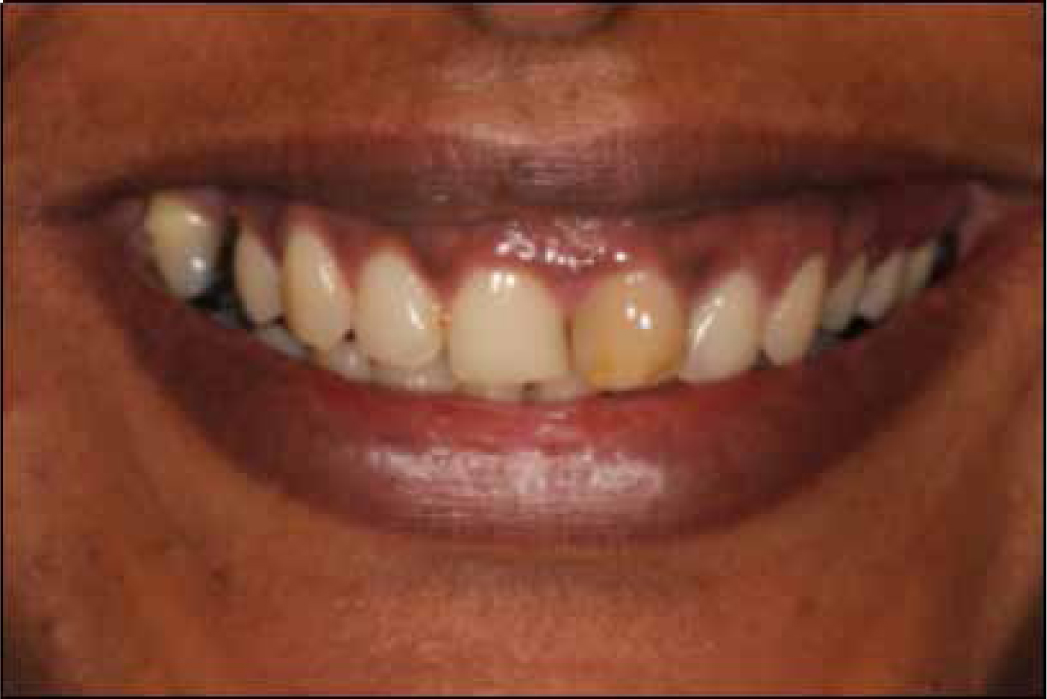
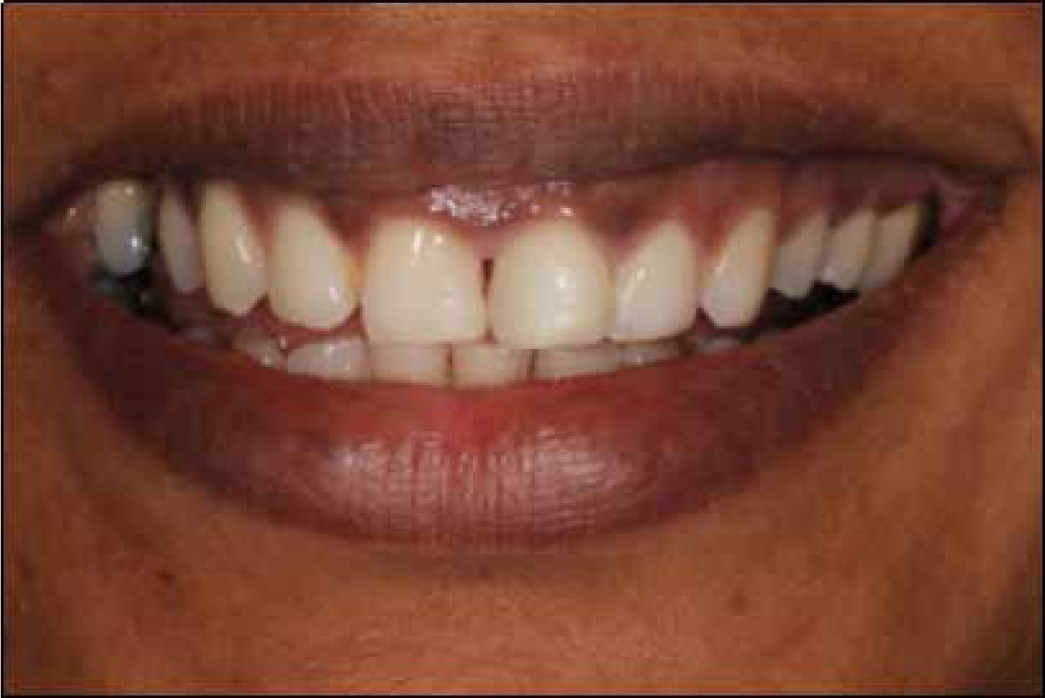
A subjective perception of tooth discoloration, or of having an unattractive natural tooth colour, can entice a patient to seek aesthetic enhancing procedures such as tooth bleaching. This safe1 treatment was popularized by Haywood and Heymann in 1989.2 They published a protocol on placing carbamide peroxide in custom-fabricated trays to lighten teeth.2 Today, the basic principles remain the same, although there have been many modifications to the procedure. The active ingredient in bleach is hydrogen peroxide (H2O2), either contained or released. There are several products available on the market and these differ in flavour, stabilizers, anti-sensitive agents, delivery, storage and marketing of the product. Regardless of who supplies the H2O2, if it is kept on the teeth for long enough whilst also adhering to a safe protocol, most teeth will bleach.
Methods available for bleaching teeth include the following.
Legality in the UK
In the UK, tooth bleaching has been associated with controversy, mainly in terms of its alleged legality, treatment provider and the concentrations of bleach that can be used.
In the case of Optident Limited and Ultradent Productions Inc vs. The Secretary of State for Trade and Industry and The Secretary of State for Health (June 2001), the House of Lords deemed tooth bleaching to be covered by the EU Cosmetics Directive (implemented in the UK via the cosmetic products safety regulations) and not the Medical Devices Directive. At the end of a tortuous series of judgments, the House of Lords concluded that bleaching of teeth was mainly used for aesthetic enhancement and not the cure of disease. This in turn meant that the EU Directive set in 1976, which stated that cosmetic products could not contain more then 0.1% hydrogen peroxide, was still applicable.3 This led to several problems. Companies were not allowed to supply bleaching products containing more than 0.1% hydrogen peroxide and, if caught doing so, could, in theory, be prosecuted by UK Trading Standards officers. Much to the bemusement and frustration of the profession, a dentist caught using more than 0.1% could theoretically face a GDC fitness to practise hearing for using a safe, evidence-based clinical procedure and, equally, clearly acting in the best interests of his/her patient, as recommended by the GDC to do so.4 Fortunately, no such prosecution occurred. Additionally, the ruling also opened the way for non-dentists to offer tooth bleaching treatment, leading to the emergence of salon- and shopping centre-based treatments, and ultimately resulting in confusion about whether tooth bleaching was the practice of dentistry. In May 2013, following a high profile case, the UK High Court ruled ‘that teeth whitening treatment comes within the practice of dentistry as identified in section 37 of the Dentists Act 1984.’ This made it illegal for anyone other than registered dentists, hygienists, or therapists under the supervision of a dentist, to practice bleaching.5
It was evident that the heart of the problem lay within the 1976 EU Council Directive on cosmetic products (76/768/EEC). Many groups and individuals had lobbied for an amendment for many years, which was finally published in September 2011 (2011/84/EU). This amendment merely added to the legal confusions that had marred bleaching for many years but meant that the law regarding UK cosmetic product safety had to change, which it did on October 2012. The new Cosmetics Products Directive listed a number of requirements that needed to be fulfilled before bleaching could be lawfully satisfied in the UK.
The Directive states that ‘over-the-counter’ bleaching products, such as mouthrinses, toothpastes or dedicated tooth bleaching products cannot contain more then 0.1% hydrogen peroxide.6 This concentration is too low to cause any significant colour change, but it does not stop manufacturers claiming that it does, or stop consumers buying the product in the belief that it will bleach their teeth.
Products containing between 0.1% and 6% hydrogen peroxide can only be supplied to a dentist and therefore only available to a consumer via the dentist. However, a number of conditions must be satisfied first.
‘An appropriate clinical examination is carried out’. A thorough clinical examination must initially be conducted, to ensure that the patient is free of any dental pathology, and to identify any risk factors that might affect the bleaching process. A thorough medical history is required. Though there are few contra-indications to the process, bleaching should probably not be undertaken in pregnant and lactating women. Although there is no evidence to suggest harm to a mother, foetus or newborn/infant, a study to establish this would be absurd. Anecdotally, it may be preferred to wait until the child is born, or, for breastfeeding mothers, until breastfeeding has discontinued. Patients diagnosed with Glucose 6-Phosphate dehydrogenase deficiency and Acatalasemia, which are rare inherited conditions, and affect the metabolism of H2O2, should avoid bleaching; or if bleaching is requested by such a patient, and use is deemed in his/her best interests for identifiable reasons, then a very mild form of peroxide should be used.7
‘Exposure to bleaching products is limited so as to ensure that the products are used only as intended in terms of frequency and duration of application.’
To prevent misuse, the dentist must ensure that the patient can apply the product safely and is aware of the consequences of over-use. Verbal instructions, a detailed instruction sheet, and a chairside demonstration would aid in satisfying this criterion. This will also satisfy another condition of the Directive which states, ‘For each cycle of use, the first use should be limited to dental practitioners, or under their direct supervision, if an equivalent level of safety is ensured.’ Essentially, for the first use, the dentist must ensure that the tray is fit for purpose, place bleach in the tray and show the patient how to place the tray and remove any excess. This is also true for patients who are to undergo another cycle of bleaching. The dentist must ensure that the patient attends for an appropriate clinical examination prior to commencement of bleaching, at which point the existing trays must be assessed and passed fit for continued use before bleach is supplied. Receptionists can no longer just supply the bleach. As mentioned above, the Directive stated ‘under direct supervision’; this means that a suitably trained hygienist or therapist can provide bleaching, even for the first cycle of use, but only as long as it is prescribed by the dentist and safety can be ensured. Some indemnity providers advise that a dentist is on the premises when the first cycle is administered by the hygienist or therapist.
‘The bleaching process should not be done in patients under the age of 18.’
The Directive only allows the bleaching process for those aged 18 or over. Under-18s who seek bleaching for purely aesthetic reasons can therefore only be treated with the wholly ineffective concentration of 0.1% of H2O2. If a patient under 18 has his/her teeth bleached, the operating dentist could potentially face a prison sentence not exceeding 6 months, a £5,000 fine or – in the worst-case-scenario – both,8 and a fitness to practise hearing at the GDC. This can pose an ethical dilemma for the dentist when presented with a discoloured anterior tooth or, more pertinently, multiple discoloured teeth, in a teenager who may be affected socio-psychologically as a result. Is the dentist still not to treat the problem teeth? Should we not act in a patient's best interest, as ordered by the GDC? Fortunately, in May 2014, the GDC clarified their stance, stating that dentists can bleach under 18s without fear of prosecution, providing it is carried out for treating or preventing disease and not for elective cosmetic reasons.9 However, it is still advised that dentists seek the opinion of their indemnity provider before providing such treatment in under 18s. It is still somewhat perplexing that one can legally provide destructive elective treatments, such as ceramic veneers, crowns or even extractions for this group, but the scientifically proven-to-be-safe and non-destructive dental bleaching is precluded by UK law.
‘An appropriate labelling regarding the concentration in hydrogen peroxide of the tooth whitening or bleaching products containing more than 0.1 %.’
Manufacturers should ensure correct labelling of all bleaching products supplied.
It is good practice when undertaking any tooth bleaching procedure that a consent form is utilized. It may help to ensure that patients understand the treatment and associated benefits and risks. Contemporaneous notes should be recorded and previous bleaching episodes enquired about.
The Directive states a concentration of greater than 6% hydrogen peroxide cannot be used. This limits the use to 6% H2O2 and 10% and 16% carbamide peroxide. 10% carbamide peroxide breaks down to 3.33%, H₂O₂ and 16% breaks down to 5 to 6%.10 However, a quick internet search provides a plethora of shops that will distribute bleaching products, sometimes in excess of 30% to any consumer, regardless of age. Patient education is important in preventing this much more dangerous concentration of bleach being directly purchased by the consumer.
If the above law is breached, then criminal prosecutions are not undertaken by the GDC but by Trading Standards. The GDC will deal with fitness to practise associated with any breach by a dentist.