References
Advice for festive drinkers
From Volume 44, Issue 11, December 2017 | Pages 1076-1082
Article

The incidence of tooth wear is rising in the UK,1 and a recent survey of 3187 young adults (aged 18 to 34 years) in seven countries in Europe concluded that ‘facial and oral tooth wear was common and affected more than 25% of this population’, with these young adults being most affected in the UK,2 with regular consumption of fruit and repeated vomiting being associated with the highest levels of tooth wear. In addition, Spijker and colleagues,3 in a literature review covering research from 10 different countries, concluded that the percentage of adults with severe tooth wear increases from 3% at the age of 20 years to 17% at 70 years. While there are a number of factors which may be implicated in this, the erosive effects of a wide variety of drinks have been identified as being increasingly relevant, with Milosevic4 presenting a list of potentially erosive drinks and foods which included:
The work of Rees and colleagues has contributed much to our knowledge of drinks which may cause erosion, given that tooth enamel has been reported to be dissolved in the presence of drinks with a pH of less than 5.5 Among the drinks tested by these researchers have been herbal teas, with the authors, Phelan and Rees,6 concluding that ‘many of the herbal teas tested were more erosive than orange juice’. In that regard, results of work by Brunton and Hussain7 indicated that herbal tea (Twinings Blackcurrant, Ginseng and Vanilla) resulted in erosion of dental enamel and that the erosive effect of this herbal tea was five times more severe than conventional black tea. Sports drinks have also been tested, by Rees, Loyn and McAndrew,5 some of which had a pH as low as 3.16. Most recently, Reddy and co-workers8 have tested the pH of 379 non-alcoholic, non-dairy drinks in stores in Birmingham, Alabama, United States, finding that 93% had a pH of less than 4.0 and concluding that ‘most are potentially erosive to the dentition’. A selection of these beverages is presented in Table 1.
| Name of Drink | pH |
|---|---|
| Lemon juice | 2.25 |
| Ocean Spray cranberry | 2.56 |
| Barber's orange juice | 3.61 |
| Minute Maid Natural Energy Mango | 3.34 |
| Juicy Juice apple | 3.64 |
| Tropicana grape juice | 3.29 |
| Perrier carbonated mineral water | 5.25 |
| Simply Lemonade | 2.61 |
| Coca Cola Zero | 2.96 |
| Coca Cola Classic | 2.37 |
| Coca Cola Cherry | 2.38 |
| Pepsi | 2.39 |
| Pepsi Max | 2.74 |
| Dr Pepper | 2.88 |
| 7UP | 3.24 |
| 7UP Diet | 3.48 |
| Red Bull regular | 3.43 |
White wines have been tested by Rees, Hughes and Innes,9 with these authors concluding that ‘many of the white wines tested had erosive potential, that there was large variation in white wines and that this could be useful information while counselling patients'. In that particular experiment, only one sparkling white wine was tested (Cava), results indicating that it had the lowest pH (<3) and the highest neutralizable acidity among the eight white wines tested, this also being greater than orange juice. Given that at a time of festivity, be this weddings, birthdays or religious festivals, people may celebrate by drinking a drink containing bubbles (be they naturally occurring as a result of the fermenting process associated with the drink, or added in the manufacturing process, both being carbon dioxide), it may be considered that there has been a paucity of testing of drinks of this nature. It is therefore the aim of this paper to test the acidity (by way of pH) and the neutralizable acidity, also known as titratable acidity (ie the volume of sodium hydroxide which is needed to be added to the drink to render its pH neutral) of a variety of alcoholic and non-alcoholic drinks containing ‘bubbles’.
Methods
A pH probe (PH0065, Jenway, UK) was attached to a pH meter (Ф40, Beckman, UK) and this was calibrated. Stock solutions of 0.1 N sodium hydroxide (NaOH) were prepared using NaOH pellets (VWR International, US) in ultra pure water (E-POD, Milli-Q, Merck, UK), and 0.1 N hydrochloric acid (HCl) from concentrated HCl diluted in ultra-pure water. The normality of the NaOH stock solution was checked and standardized against HCl solution by titrating NaOH against HCl. A phenol red indicator solution (P/2420, Phenol Red, Fisons Scientific App Ltd, UK) was prepared in ethanol (1 mg/50 ml) and 3 drops were added to the beaker. The pH range for the indicator solution was pH 6.8–8.4 and was indicative of a colour change from yellow to red (Figures 1 and 2).


A magnetic stirrer bead was placed into the solution and the solution was stirred at 300 rpm constantly at room temperature using a magnetic stirrer hot plate. A 50 ml graduated, self-zeroing, calibrated burette was filled with 0.1 N NaOH and the stopcock was opened so the NaOH was released into the stirring HCl solution dropwise. The NaOH was titrated against HCl until a permanent yellow to red colour change was achieved and the reading from the burette at the meniscus was recorded. The volume of NaOH used was calculated.
The drinks which were tested are listed in Table 2. Three bottles of each beverage were stored overnight in the same refrigerator (Whirlpool Type REI30A) in order to ensure that they were at the same temperature (4 °C). Titrations were performed on three 10 ml aliquots of each bottle to compare batch to batch variation. The titrations for the drinks were performed using the same protocol as described for the standardization using HCl except that, for each drink titration, the pH probe was introduced to the stirring solution to monitor the pH of the drink solutions. For each titration, pH values were recorded before and after titration. Since colour change may be difficult to assess in some coloured drinks, the titration endpoint was standardized to pH 8.2.10 The total acid relative to tartaric acid, malic acid and citric acid was calculated from the volume of NaOH used according to the following equation:
| ▪ Sparkling water (Aqueo, Chase Spring, Lichfield, Staffordshire, UK); |
| ▪ Sparkling natural mineral water (Badoit, Saint Galmier, France); |
| ▪ Tonic water (Schweppes); |
| ▪ Slimline Tonic Water (Schweppes); |
| ▪ Bucks Fizz (Winemakers selection by Sainsburys) 4% vol; |
| ▪ Shloer non-alcoholic sparkling white grape juice; |
| ▪ Älska Nordic Berries Cider (The Swedish Cider Company, Stockholm) 4.0% vol; |
| ▪ Orchard Premium Irish Cider 4.5% vol; |
| ▪ Asti Vino Spumante Dolce (S Orsola, Italy) 7% vol; |
| ▪ Prosecco Extra Dry (Valdobbiadene, Italy) 11% vol; |
| ▪ Champagne Monsigny Brut (Philizot et fils, France) 12% vol; |
| ▪ Lanson Brut Rosé (Reims, France) 12.5%; |
| ▪ Saumur Rosé Brut (Bouvet, Saumur) 12.5%; |
| ▪ Soda Water (Marks and Spencer). |
Total Acid = Volume of NaOH used ml x Normality of NaOH x Acid Constant
Multifactorial analysis of variance (ANOVA) and post hoc Tukey tests (P < 0.05) were performed on the data sets to compare the differences between drinks and batches (Minitab, Version 17, UK).
Further details of the exact methodology are available from the first author on request.
Results
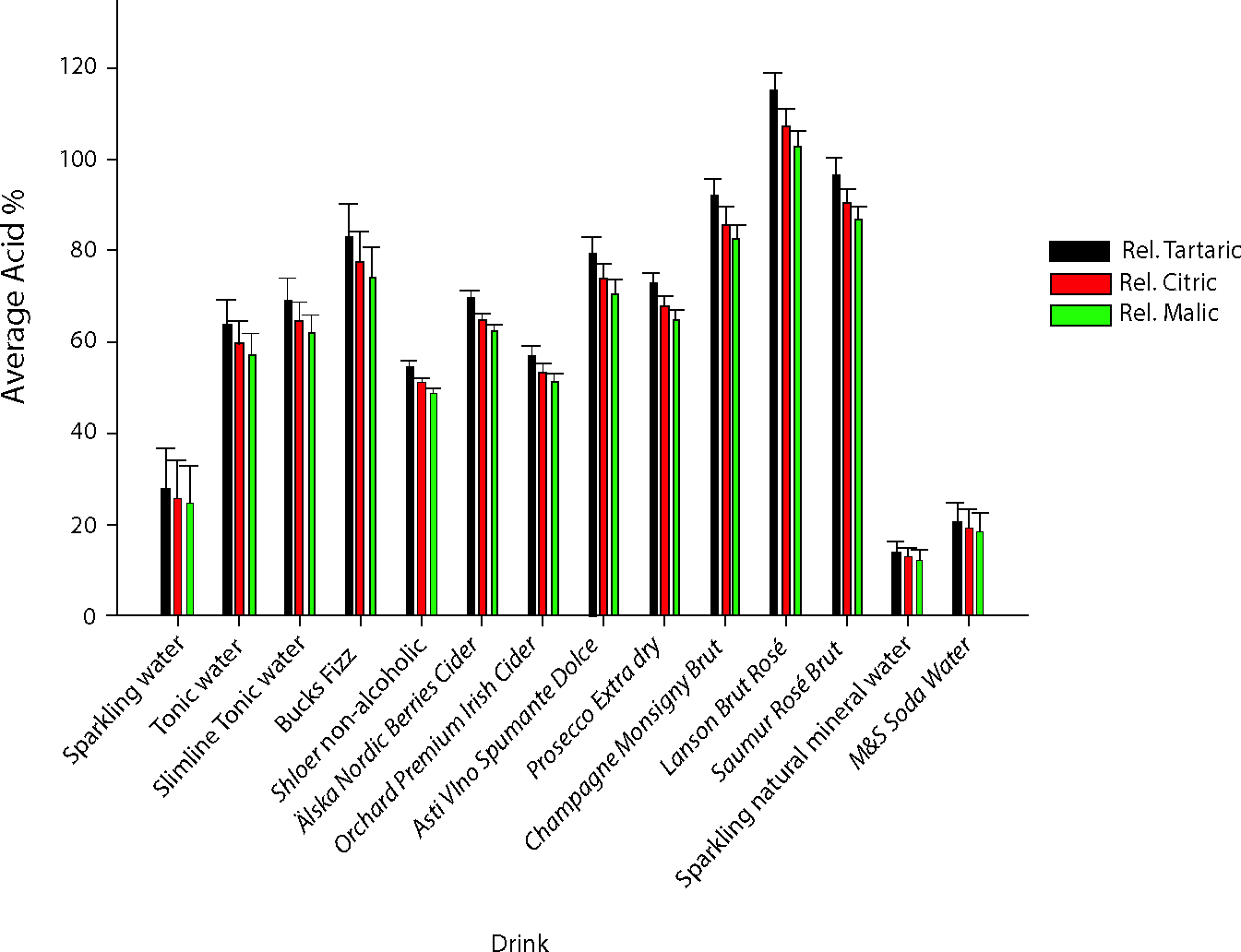
The General Linear Model (GLM) ANOVA identified significant differences in pH and titratable acidity for each of the drinks tested (P < 0.05). The GLM analysis also revealed significant differences between batches for titratable acidity but not for pH. Figure 3 shows the average pH of each drink tested and Table 3 reports the one-way ANOVAs and post-hoc Tukey comparisons. The pH of the drinks varied significantly (P < 0.05) and ranged between Schweppes Tonic Water at 2.54 and Sparkling Mineral Water at 6.21 (Figure 3 and Table 3). The titratable acidity also significantly varied between drinks, with the highest titratable acidity being obtained for Lanson Brut Rosé champagne and the lowest for Sparkling natural mineral water (Figure 4 and Table 3). A weak exponential inverse relationship was measured between pH and titratable acidity where increasing titratable acidity was characteristic of decreasing pH (R2= 0.8757; Figure 5).
| Drink | pH (start) | vol. NaOH used (ml) | Acid% (rel. tartaric acid) | Acid% (rel. citric acid) | Acid% (rel. malic acid) |
|---|---|---|---|---|---|
| Sparkling water | 4.93 (0.13)c | 3.67 (1.24)i | 27.5 (9.29)i | 25.67 (8.67)i | 24.57 (8.3)i |
| Tonic water | 2.54 (0.06)j | 8.56 (0.82)f,g | 63.79 (5.51)f,g | 59.54 (5.14)f,g | 56.98 (4.92)f,g |
| Slimline tonic water | 3.16 (0.00)e,f | 9.23 (0.60)e,f | 69.25 (4.5)e,f | 64.64 (4.2)e,f | 61.87 (4.02)e,f |
| Bucks Fizz | 3.38 (0.01)d | 11.03 (0.98)c | 83.03 (7.4)c | 77.49 (6.91)c | 74.17 (6.61)c |
| Shloer non-alcoholic | 3.18 (0.01)e | 7.30 (0.13)h | 54.75 (1.0)h | 51.1 (0.93)h | 48.91 (0.89)h |
| Älska Nordic Berries Cider | 3.08 (0.01)g | 9.46 (0.20)d,e,f | 69.78 (1.44)e,f | 65.13 (1.35)e,f | 62.33 (1.29)e,f |
| Orchard Premium Irish Cider | 3.11 (0.01)f,g | 7.76 (0.29)g,h | 57.23 (2.12)g,h | 53.42 (1.98)g,h | 51.13 (1.89)g,h |
| Asti Vino Spumante Dolce | 2.91 (0.00)h | 10.51 (0.49)c,d | 79.06 (3.72)c,d | 73.79 (3.47)c,d | 70.62 (3.32)c,d |
| Prosecco Extra Dry | 2.89 (0.02)h | 9.70 (0.27)d,e | 72.99 (2.01)d,e | 68.13 (1.87)d,e | 65.21 (1.79)d,e |
| Champagne Monsigny Brut | 2.79 (0.01)i | 12.29 (0.52)b | 92.17 (3.89)b | 86.03 (3.63)b | 82.34 (3.48)b |
| Lanson Brut Rosé | 2.74 (0.01)i | 15.61 (0.54)a | 115.2 (3.95)a | 107.52 (3.69)a | 102.91 (3.53)a |
| Saumur Rosé Brut | 2.87 (0.01)h | 13.13 (0.47)b | 96.91 (3.44)b | 90.45 (3.21)b | 86.57 (3.08)b |
| Sparkling natural mineral water | 6.21 (0.05)a | 1.85 (0.34)j | 13.65 (2.54)j | 12.74 (2.37)j | 12.2 (2.27)j |
| M&S Soda Water | 5.50 (0.04)b | 2.73 (0.64) | 20.4 (0.05)i.j | 19.0 (0.04)i.j | 18.2 (0.04)i.j |


Discussion
pH is a logarithmic measure of the concentration of free hydrogen ions in a chemical or biological system. Titratable acidity is a simple measure of the relative amount of acid anions in a sample. Titratable acidity in grape juice or wine by convention is expressed as total acids (g/L) or percentage of tartaric acid, although the total acidity is given by a mixture of acids, predominantly tartaric and malic, but also citric, succinic and trace amounts of other weak and strong acids. The advantage of measuring titratable acidity using a pH meter (known as the potentiometric method) is that the precise equivalent point can be identified. However, the limitations of such a method, including the physical constraints of the pH probes and slow responses with some electrodes, makes measurements cumbersome. Nonetheless, since the acid profile of a given wine, juice or other beverage is unique, the true equivalence point will vary with each individual beverage and the accepted convention in food and drink analysis is to use a standardized endpoint of pH 8.2 (the phenolphthalein endpoint) to allow comparisons.10 Whilst pH 7 marks the point of neutrality on the pH scale, this is not typically chosen as an endpoint since, once all the acid has been neutralized, the conjugate base remains. Thus, the equivalence point is slightly greater than 7.
Here, both pH and titratable acidity (relative to common acids in drinks) were measured. A weak exponential inverse relationship between pH and titratable acidity was measured (R2 = 0.8757) where pH decreased with increasing titratable acid content. However, generally, there is no direct or predictable relationship between pH and titratable acidity, since pH is a measure of the amount of free hydrogen ions (ie the ability of acids to dissociate which can be considered as its strength) and titratable acidity is a measure of the total amount of hydrogen ions (the concentration of acids present). Thus it is possible that different pH values can be measured for drinks with the same titratable acidity. Here, the drinks are likely to be made up of different types of acids, having different strengths and concentrations. Since titratable acidity is a measure of the amount of acid in a solution and pH is the strength, some drinks may contain smaller quantities of strong acids, which will result in low pH (ie more acidic) and low titration values, whereas other drinks may contain higher concentrations of weak acids, which will result in higher pH (ie less acidic) and higher titration values. It is important to address both of these factors when considering the potential damage which drinks may cause to teeth. In addition, there has been recent publicity regarding the sugar content of Prosecco11 and the potential for dental caries among drinkers of this beverage.
The drinks were stored in a refrigerator prior to testing, as it has been demonstrated, for some erosive drinks, that there is an inverse relationship between material loss and surface hardness as temperature increased, another potential lesson for festive drinkers.12
Some advice for festive drinkers
The results present challenging reading for those who indulge in a variety of sparkling drinks during festive times, given that the pH of many was considerably below the pH at which enamel is known to dissolve (and readers are reminded that pH is a logarithmic scale). In this regard, as the pH drops, the surface erodes, and with each unit of decrease in pH, there is a 10-fold increase in enamel solubility.13 Indeed, the only drinks which did not have any potential for tooth erosion, from the drinks selected, were the three varieties of sparkling water/soda water. However, not all readers will classify these as ‘festive’ drinks. The six sparkling drinks containing alcohol, the non-alcoholic drink (Shloer) and the two ciders all had pH values in the region of 3, while the two rosé alcoholic drinks, the Lanson Rosé and the Saumur Rosé had higher titratable acidity than any of the other drinks, a worry perhaps for those festive drinkers who prefer the grape skins to have touched the grape juice for a short time prior to fermentation. More work therefore appears to be indicated, on whether sparkling rosé wines, in general, are more likely to be erosive than those which are white.
Of course, the potential for tooth erosion for the champagnes and other sparkling drinks tested relates not only to their acidity, but also to the volume consumed. The advice, from the results of our work, therefore, for festive drinkers is that high consumption of these drinks at festive times may have a deleterious effect on their enamel, and ultimately their dentine, let alone their general health if high volumes of alcoholic beverages continue to be consumed.
Comparing the titratable acidity of carbonated beverages in this study with other ‘flat’ beverages, it is clear that the absence of carbon dioxide may reduce the potential challenge to the natural dentition. The results of the present study would therefore tend to suggest that party-giving practitioners who are concerned about their guests' enamel, but determined for social reasons to serve festive drinks such as Champagne and Shloer, might be well advised to ensure that the bottles are opened the day prior to the party so that the carbon dioxide can escape from the solution. Carbon dioxide solubility at 30 °C is ten times less than at 8 °C,14 so further reduction may be achieved by warming the bottles up, for example on a radiator, immediately before serving! There is a possibility that such measures in themselves may decrease the amount of consumption that occurs by rendering the previously quaffable drinks undrinkable, and if these recommendations are widely adopted there may follow clear dental public health (indeed, overall public health) benefits. Further controlled trials are needed.
Conclusion
Of the drinks tested, other than the sparkling mineral waters, drinks containing bubbles may be damaging to teeth via an erosive effect, depending on the volumes consumed at festive, and other, times.
