The immune system: basis of so much health and disease: 8. antigens and MHC
From Volume 44, Issue 11, December 2017 | Pages 1071-1075
Article



Antigens
Antigens (immunogens) are substances, usually proteins, that can be recognized by the body as foreign and can elicit an antibody response. Antigens are usually exogenous but may be endogenous (host-derived). An epitope, or antigenic determinant, is the part of an antigen recognized by the immune system, specifically by antibodies, B-cells, or T-cells.
Exogenous antigens
Exogenous antigens are often microbial; bacterial antigens include:
Viral antigens include:
Fungal antigens include:
Endogenous antigens
Endogenous antigens include human tissue antigens, such as:
Super-antigens
Super-antigens (SAgs) have the following features:
Haptenes
Haptenes are low molecular weight substances which, though not on their own immunogenic, become immunogenic if coupled to a larger carrier molecule (eg albumin, globulins). Examples of haptenes include chemicals and drugs (eg penicillin, sulphonamides, aspirin, cosmetic, tranquillizers, and neomycin).
The properties of antigens are summarized in Table 1.
| Immunogenicity | Ability to induce an immune response (humoral and/or cellular) when they enter into the organism |
| Antigenicity | Specific binding ability to the end products of the immune response, regardless of immunogenicity |
| Allergogenicity | Ability to induce allergic reactions |
| Tolerogenicity | Property of antigens to suppress specific immune response |
The major histocompatibility complex
The major histocompatibility complex (MHC), also referred to as the human leukocyte antigen (HLA) complex, is a generic name for the gene complex that codes the self antigens important in T-cell recognition of foreign antigens.
The MHC is divided into three regions designated:
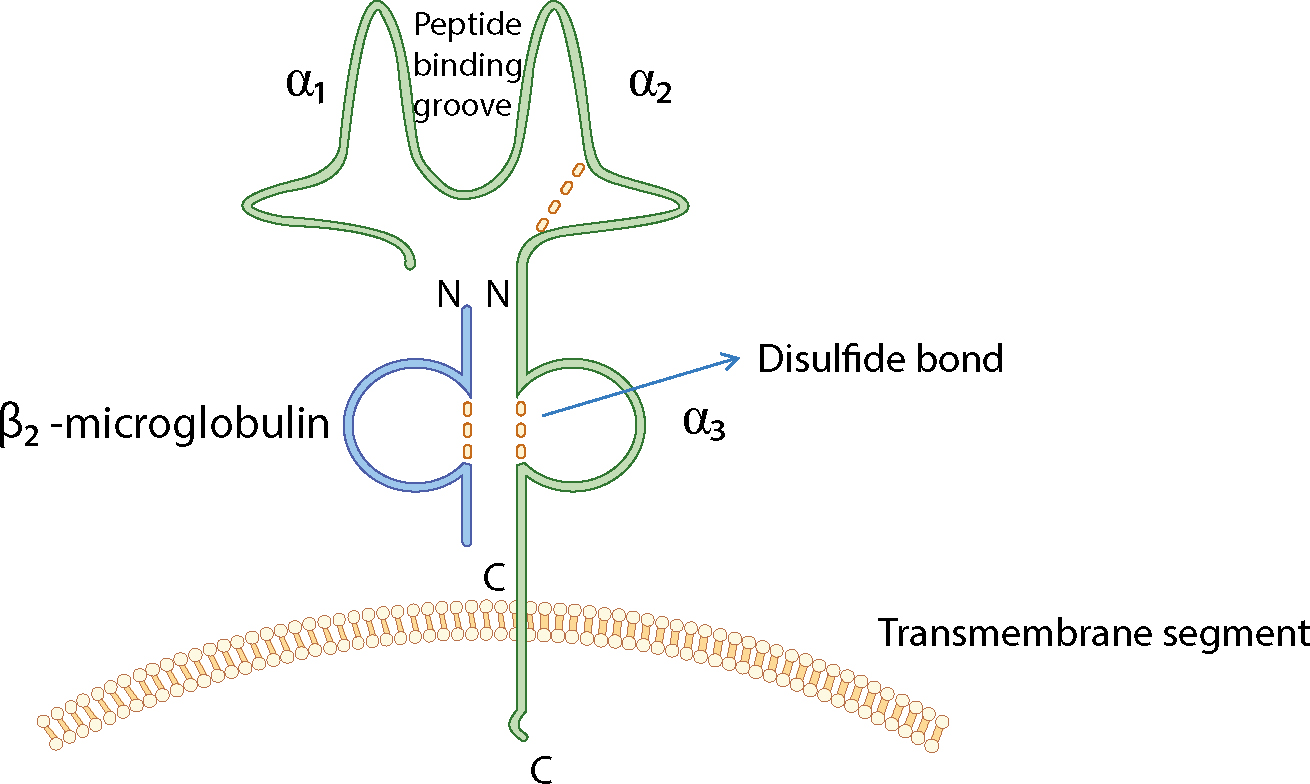
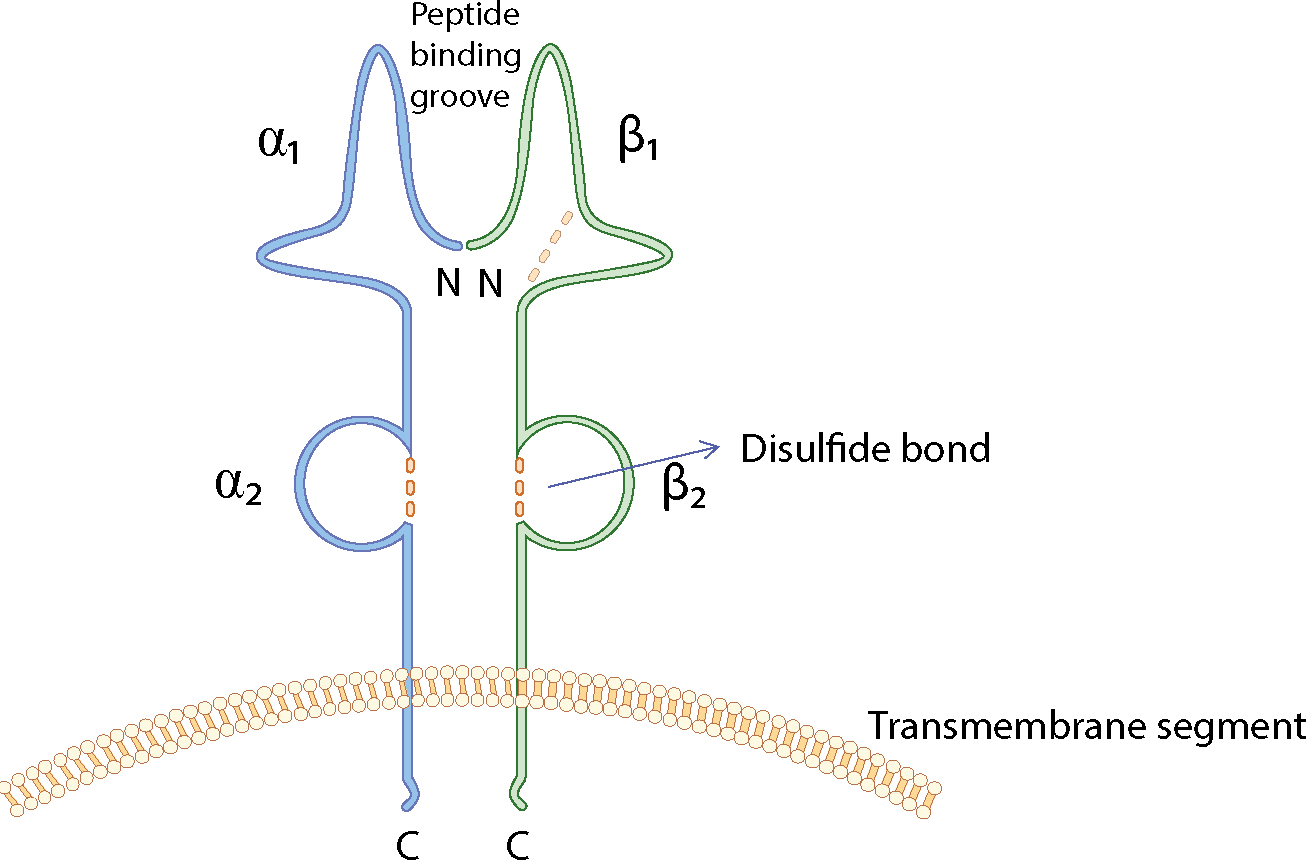
The loci that make up the MHC are highly polymorphic (many alternate forms of the gene [alleles] exist at each locus) and are closely linked (they tend to be inherited as a block, called a haplotype). Features of the MHC class molecules are summarized in Table 2.
| MHC Class | Present on | Effect | |
|---|---|---|---|
| I | HLA A,B,C | Most cells | With antigen, signal CD8 cytotoxic T-cells to destroy their carrier cell |
| II | HLA DR, DP | B-cells, macrophages, endothelial cells | Signal to CD4 T-cells |
Antigen presentation
The basis of acquired immunity is the capacity of immunocytes to distinguish between self cells, and foreign antigens – usually infectious pathogens. Certain cells capture antigens and enable their recognition by various other cells. This process is termed ‘antigen presentation’ (Table 3).
| Macrophage | Dendritic Cell | B-cell | |
|---|---|---|---|
| MHC-II Expression | Low levels. |
Always expressed | Always expressed |
| Antigen Type and Presentation by MHC | Extracellular antigens: presentation via MHC-II | Intracellular and extracellular antigens: presentation via MHC-I and II | Extracellular antigen binds to specific Ig receptors: presentation via MHC-II |
| Co-Stimulation (B7 Expression) | Low levels |
Always expressed at high levels | Low levels |
| Location | Lymphoid tissue |
Lymphoid tissue |
Lymphoid tissues Blood |
Many cells are capable of presenting antigens and activating acquired immunity but some are specially equipped to acquire and present antigen, and to prime T-cells. These are the antigen-presenting cells (APCs). APCs are mainly macrophages, or dendritic cells such as:
APCs capture and process antigens, and migrate to local lymphoid tissue, and then present the antigen in association with MHC molecules to engage with and activate T-lymphocytes, to initiate a local immune response.
Most cells can present antigen to CD8+ T-cells via MHC Class I molecules and are thus ‘APCs’. APCs that express MHC Class II molecules as well as MHC Class I molecules are often called professional antigen-presenting cells, and can stimulate helper T-cells as well as cytotoxic T-cells. The professional APCs also express co-stimulatory B7 molecules, and include:
Dendritic cells
Dendritic cells:
APCs are activated by signalling though TLRs (toll-like receptors), generating pro-inflammatory cytokines which control the subsequent killing of invading pathogens or of virally-infected cells.
Macrophages
Macrophages are part of the innate immune response, continuously phagocytosing self-proteins and cells in their proximity. All of these antigens are degraded and presented on MHC-II, but do not activate T-cells, because only low levels of MHC-II, and almost no co-stimulator (B7), are expressed. Macrophages have receptors that recognize carbohydrate patterns on foreign cells such as micro-organisms and for specific bacterial products such as lipopolysaccharide (LPS) (endotoxin). If these macrophage molecules bind their bacterial ligands, MHC-II and B7 are up-regulated, increasing antigen presentation and cytokine (IL-1, IL-6, IL-8, IL-12 and TNF-alpha) secretion, activating Th-cells.
B-lymphocytes
B-lymphocytes express antigen receptors, which are required for antigen recognition, though B-cells can recognize some antigens in the absence of antigen presentation.
B-cell receptors (BCRs):
Antigen recognition
T-cells recognize antigens but only (with the important exception of superantigens) if there is proper antigen presentation – peptide fragments which have been processed and bound to MHC molecules. This is known as MHC-restricted specificity. In other words, it is essential that dendritic cells are genetically identical to the responding T-cells; simple diffusion of unprocessed antigen to lymph nodes and T-cells is not immunogenic.
Different types of antigen are presented differently from T-cells: there are two pathways for such antigen presentation (endogenous and exogenous).
Endogenous pathway
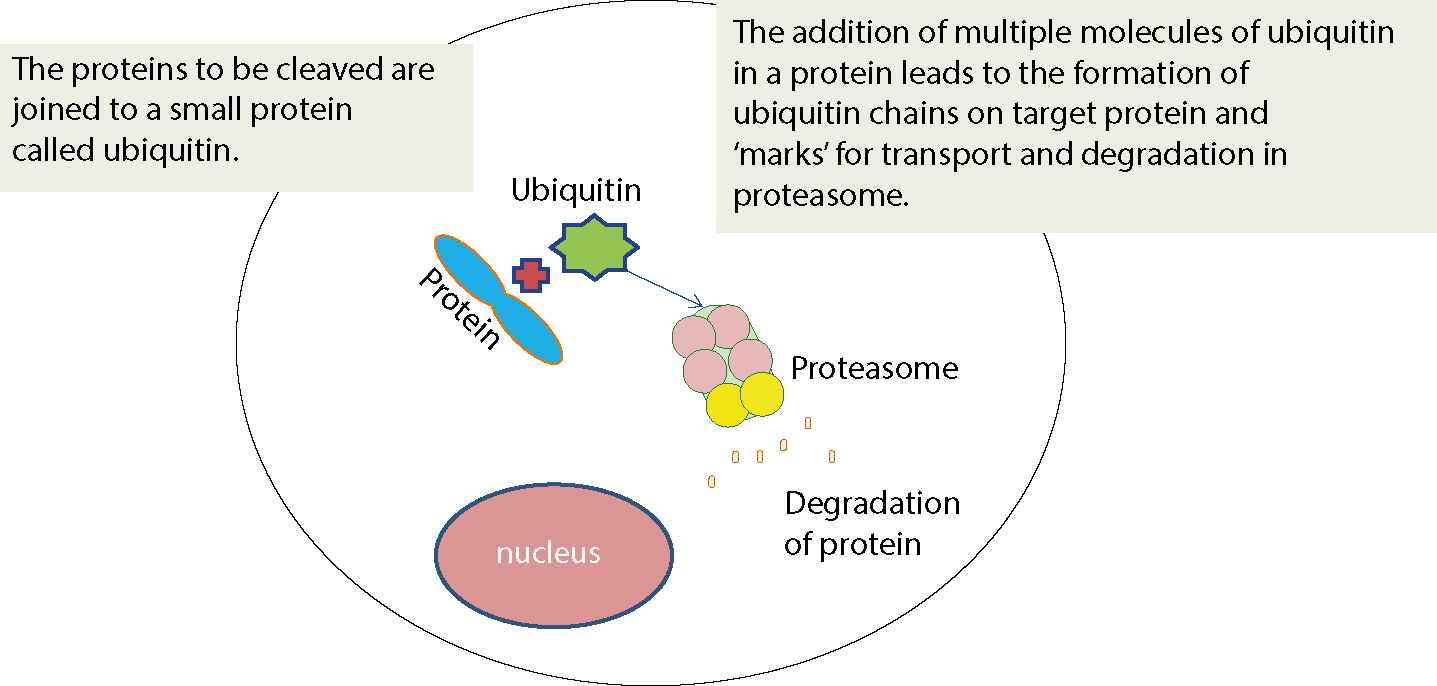
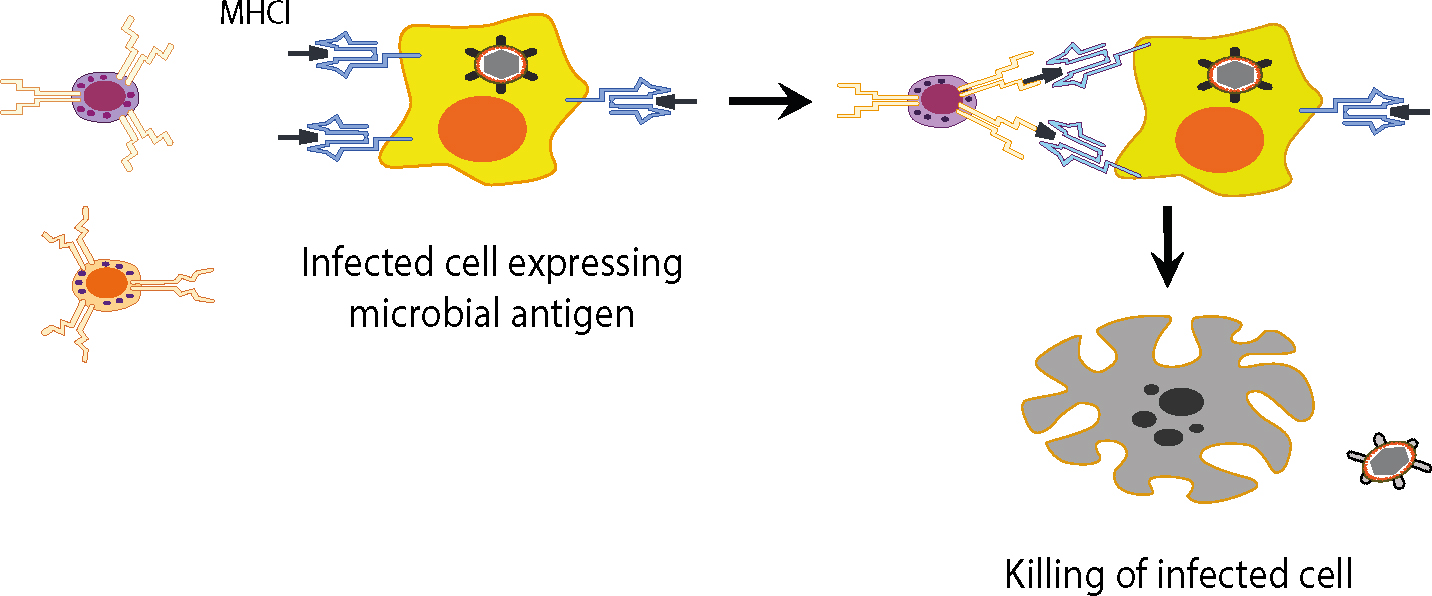
In the endogenous pathway, pathogens that enter the cell cytosol (ie viruses, some bacteria) are fragmented and their peptides are bound to MHC Class I proteins for presentation to T-cells. After a virus enters a cell and uncoats, it enters the nucleus, integrates with host DNA and replicates, viral proteins appearing in the cytosol, then to be degraded into peptides by proteasomes. Such peptides are then transported into the rough endoplasmic reticulum for loading onto MHC Class I molecules. The peptide-MHC complex is transported to the cell surface where it can be recognized by the T-cell receptor (TCR) of an MHC Class I-restricted T-cell. The Class I MHC-molecules bind peptides derived from the degradation of endogenous proteins (physiological, or aged or defective cell proteins, viruses, parasites and tumour proteins). Thus, for example, when a virus enters a cell it can use the cell biosynthetic machinery for the expression of genes and the production of viral proteins. Accordingly, almost every cell in the body is an ‘open book’ which gives information to the T-lymphocytes for the kind of proteins present in the interior. Various defective driver or aged or various regulatory proteins that have completed their work and whose prolonged stay in the cytoplasm or the nucleus could disrupt the orderly functioning of the cell ‘are marked’ in order to identify and bring them to the organelle that will deconstruct them. This is achieved by linking them to a specific protein (ubiquitin). The cells destroy proteins to lysosomes in the cytoplasm. The lysosomes contain various proteolytic enzymes. There are three types of lysosomes, catalytic enzymes having trypsin activity, chymotrypsin and peptidyl-glutamyl-peptide hydrolase (PGPH). The degradation of proteins in the cytoplasm is made continuously by means of a cluster of multicatalytic proteases which comprises 28 subunits, called the proteasome. The ubiquitin proteasome system is depicted in Figure 3.
Exogenous pathway
In the exogenous pathway, proteins are endocytosed, and pathogens in the endosomal/lysosomal system (ie most bacteria) are broken to peptides which bind MHC Class II molecules in a specialized endosomal compartment called MIIC, and are then exported to the cell surface, where the complex can be recognized by the T-cell receptor (TCR) of an MHC Class II-restricted T-cell.
APCs express co-stimulatory proteins such as CD80 and CD86 required for APCs to present antigen to T-cells.
Responding T-cells, in addition to the antigen, also receive a complementary interleukin-1 (IL-1) signal from both dendritic cells and keratinocytes which prompts activated T-cells to secrete IL-2 which binds to additional antigen-responsive T-cells causing them to proliferate, dramatically increasing the number of T-cells capable of responding to the challenge.
Activated T-cells also produce IFN-a which stimulates dendritic cells (and possibly keratinocytes) to enhance antigen presentation.
Activated T-cells evoke either a specific immunological response to the antigenic challenge via macrophages (a phenomenon again restricted by MHC-specificity) or, alternatively, activate defence cells by releasing lymphokines.
The major consequences of antigen presentation to T-lymphocytes are:
T-cell receptors (TCR) consist of α and β chains anchored to T-cells.
Co-stimulatory molecules are also essential for T-cell activation; molecules such as B7 bind to CD28 receptors on the T-cells to cause activation. Antigens presented without such co-stimulation usually induce T-cell hypo-responsiveness (anergy).
The affinity of binding of an antigenic peptide within the TCR-MHC-peptide complex determines whether or not the T-cell will be activated. High-affinity interactions promote T-cell activation: low-affinity interactions may antagonize activation.
Intercellular adhesion molecules may also contribute to the interaction between APCs and T-cells.
T-cell activation
T-cell activation induces enzyme cascades, leading to the production of IL-2 and the high affinity IL-2 receptor on the T-cell. IL-2 is required to drive T-cell division.
Mature Th-cells proliferate and differentiate into antigen-specific effector Th-cells, releasing cytokines which activate macrophages, NK and B cells – effector cells which kill targets, ie nucleated cells expressing MHC-I (infected with viruses, tumour cells or graft cells) – and memory cells which provide secondary immune response.
Activated Th-cells (Th1) secrete interferon IFN-γ, which activates macrophages and increases their ability to kill ingested intracellular pathogens and abnormal host cells (abnormal or tumour cells) non-specifically.
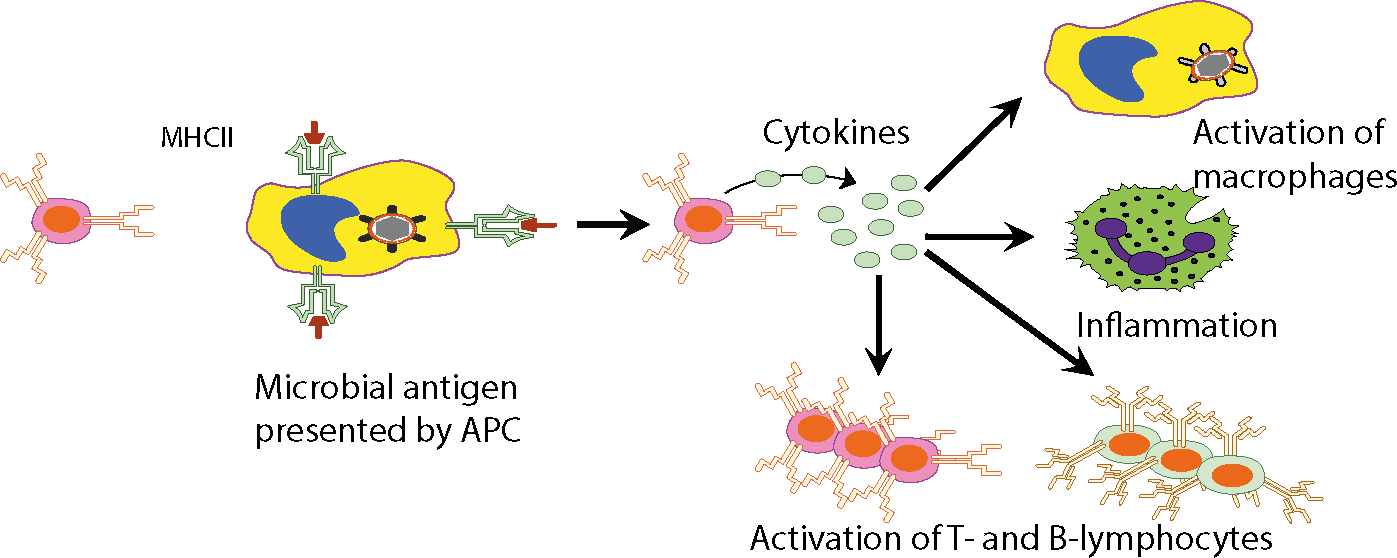
The antigen presentation and the effects that follow are depicted in Figures 4 and 5.
Conclusion
Antigens are substances recognized by the body as foreign and can elicit an antibody response.
The major histocompatibility complex (MHC), (aka human leukocyte antigen (HLA) complex), is the gene complex that codes the self antigens important in T-cell recognition of foreign antigens.
Antigen-presenting cells (APCs) are equipped to acquire and present antigen, and to prime T-cells; main APCs are Dendritic cells, Macrophages and B-lymphocytes.
